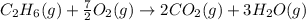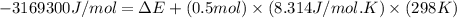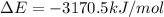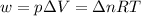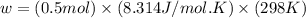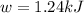Chemistry, 26.07.2019 04:00 tonytashaqua
Calculate the work (w) and δeo, in kj, at 298 k and 1 atm pressure, for the combustion of one mole of c6h6 (g). first write and balance the equation. the products will be co2 (g) and h2o (g). the value of δho for this reaction is -3169.3 kj/mol.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
Calculate the work (w) and δeo, in kj, at 298 k and 1 atm pressure, for the combustion of one mole o...
Questions

Mathematics, 02.06.2021 21:30


Mathematics, 02.06.2021 21:30

Mathematics, 02.06.2021 21:30


Mathematics, 02.06.2021 21:30

Mathematics, 02.06.2021 21:30

Social Studies, 02.06.2021 21:30





Chemistry, 02.06.2021 21:30

Mathematics, 02.06.2021 21:30

Mathematics, 02.06.2021 21:30



Mathematics, 02.06.2021 21:30


Mathematics, 02.06.2021 21:30

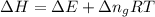
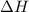 = change in enthalpy = -3169.3 kJ/mol = -3169300 J/mol
= change in enthalpy = -3169.3 kJ/mol = -3169300 J/mol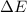 = change in internal energy
= change in internal energy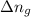 = change in moles
= change in moles