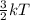Chemistry, 25.07.2019 18:50 Sparkleskeepsgoing
What happens when you increase the temperature of a reaction? a. more collisions occur and the time required for the reaction increases b. fewer collisions occur and the time required for the reaction decreases c. fewer collisions occur and the time required for the reaction increases d. more collisions occur and the time required for the reaction decreases.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2
You know the right answer?
What happens when you increase the temperature of a reaction? a. more collisions occur and the time...
Questions


Mathematics, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00




History, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00



Physics, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00

Computers and Technology, 16.12.2020 01:00

English, 16.12.2020 01:00



Chemistry, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00