

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 08:30
Benzonitrile (c6h5cn) is reduced to two different products depending on the reducing agent used. treatment with lithium aluminum hydride followed by water forms k, which has a molecular ion in its mass spectrum at 107 and the following ir absorptions: 3373, 3290, 3062, 2920, and 1600 cm-1. treatment with a milder reducing agent forms l, which has a molecular ion in its mass spectrum at 106 and the following ir absorptions: 3086, 2850, 2820, 2736, 1703, and 1600 cm-1. l shows fragments in its mass spectrum at m/z = 105 and 77. propose structures for k and l and choose an explanation for how this could be concluded.
Answers: 3
You know the right answer?
The first ionization energies of an element are approximately 738 kj/mol, 1450 kj/mol, 7.7 × 103 kj/...
Questions

Mathematics, 04.11.2019 23:31

English, 04.11.2019 23:31

Chemistry, 04.11.2019 23:31


Mathematics, 04.11.2019 23:31


Spanish, 04.11.2019 23:31



Business, 04.11.2019 23:31

Mathematics, 04.11.2019 23:31



Biology, 04.11.2019 23:31



Mathematics, 04.11.2019 23:31

Mathematics, 04.11.2019 23:31


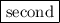 group of the periodic table.
group of the periodic table.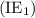 . Similarly, when the second electron is removed from the monoatomic cation, ionization energy is called the second ionization energy
. Similarly, when the second electron is removed from the monoatomic cation, ionization energy is called the second ionization energy 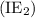 and so on…
and so on…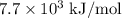 and
and 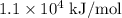 respectively
respectively

