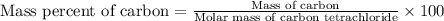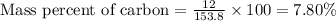Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which one of the following gases is not an important component of soil?
Answers: 2

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
Calculate the mass percent of carbon (c) in carbon tetrachloride, (ccl4), if the molar mass of carbo...
Questions











English, 10.03.2020 22:39







Mathematics, 10.03.2020 22:39


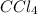 , there are 1 carbon atoms and 4 chlorine atoms.
, there are 1 carbon atoms and 4 chlorine atoms.