
Chemistry, 27.10.2019 16:43 lashaaungas
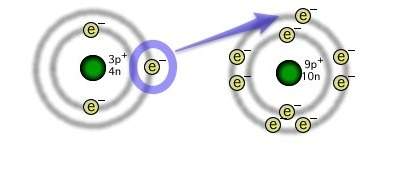
The illustration depicts the formation of an ionic chemical bond between lithium and fluorine atoms. why is the resulting compound more stable than the individual atoms?
a) the shared electron from lithium to fluorine provides each atom with a full outer energy level.
b) the shared electron from lithium to fluorine provides each atom with an empty outer energy level.
c) the transferred electron from lithium to fluorine provides each atom with a full outer energy level.
d) the transferred electron from lithium to fluorine provides each atom with an empty outer energy level.
< 3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

You know the right answer?
The illustration depicts the formation of an ionic chemical bond between lithium and fluorine atoms....
Questions


Mathematics, 09.04.2021 06:40



Mathematics, 09.04.2021 06:40

Mathematics, 09.04.2021 06:40


Mathematics, 09.04.2021 06:40

Arts, 09.04.2021 06:40



Mathematics, 09.04.2021 06:40


Mathematics, 09.04.2021 06:40






Mathematics, 09.04.2021 06:40



