
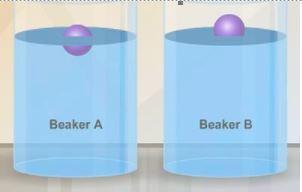
Identical objects are placed in beaker a and beaker b. the objects float as shown in the diagram. what can you conclude about the liquid in each of the beakers? a question 6 options: the liquid in beaker b has a greater density than the liquid in beaker a. the liquid in beaker a has a greater density than the liquid in beaker b. the liquid in beaker a has a greater density than the objects. the liquid in beaker b is less dense than the objects. both liquids are less dense than the objects. i think the answers are b or d me!

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
Identical objects are placed in beaker a and beaker b. the objects float as shown in the diagram. wh...
Questions

History, 29.06.2021 21:10


World Languages, 29.06.2021 21:10

History, 29.06.2021 21:10

Physics, 29.06.2021 21:10

Mathematics, 29.06.2021 21:10

Mathematics, 29.06.2021 21:10


Biology, 29.06.2021 21:10


History, 29.06.2021 21:20

Mathematics, 29.06.2021 21:20



Mathematics, 29.06.2021 21:20

English, 29.06.2021 21:20


Mathematics, 29.06.2021 21:20

Social Studies, 29.06.2021 21:20



