
Chemistry, 24.07.2019 10:30 graymonky12
Balance the following equation. then determine the ratio for the products kcl and o2 generated during the decomposition of potassium chlorate. kclo3 kcl + o2 1: 1 2: 2 4: 3 2: 3 3: 2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
Balance the following equation. then determine the ratio for the products kcl and o2 generated durin...
Questions


Mathematics, 05.02.2020 08:52

Geography, 05.02.2020 08:52


Physics, 05.02.2020 08:52


English, 05.02.2020 08:52

Mathematics, 05.02.2020 08:52

Mathematics, 05.02.2020 08:52











History, 05.02.2020 08:52

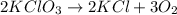
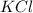 and 3 moles of
and 3 moles of 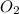 .
.

