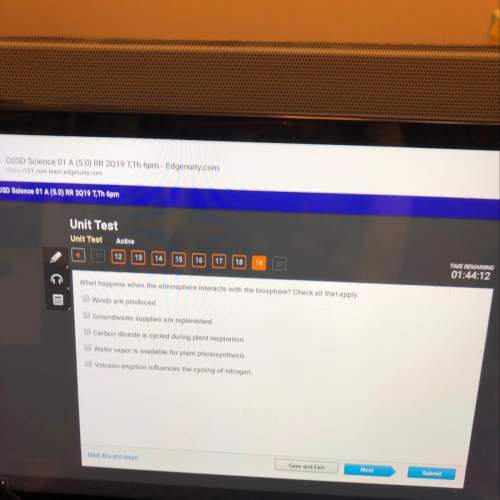
When a sample of aqueous hydrochloric acid was neutralized with aqueous sodium hydroxide in a calorimeter, the temperature of 100.0 g of water surrounding the reaction increased from 25.0°c to 31.5°c. if the specific heat of water is 1.00 cal/(g•°c), calculate the quantity of energy in calories involved in this neutralization reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral.a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

You know the right answer?
When a sample of aqueous hydrochloric acid was neutralized with aqueous sodium hydroxide in a calori...
Questions




Biology, 04.02.2020 18:52







History, 04.02.2020 18:52

Geography, 04.02.2020 18:52

Biology, 04.02.2020 18:52

History, 04.02.2020 18:52


Mathematics, 04.02.2020 18:52

English, 04.02.2020 18:52

History, 04.02.2020 18:52

History, 04.02.2020 18:52