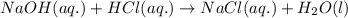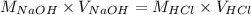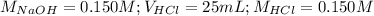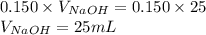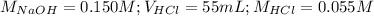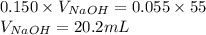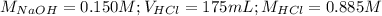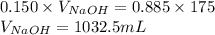Chemistry, 23.07.2019 20:10 mrylenastewart
Determine the volume of 0.150 m naoh solution required to neutralize each sample of hydrochloric acid. the neutralization reaction is: naoh(aq) + hcl(aq) → h2o(l) + nacl(aq) 25 ml of a 0.150 m hcl solution 55 ml of a 0.055 m hcl solution 175 ml of a 0.885 m hcl solution

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
Determine the volume of 0.150 m naoh solution required to neutralize each sample of hydrochloric aci...
Questions




Mathematics, 23.10.2020 18:00


English, 23.10.2020 18:00



Computers and Technology, 23.10.2020 18:00



History, 23.10.2020 18:00

Mathematics, 23.10.2020 18:00

History, 23.10.2020 18:00

Mathematics, 23.10.2020 18:00


Mathematics, 23.10.2020 18:00

Mathematics, 23.10.2020 18:00

Computers and Technology, 23.10.2020 18:00

Physics, 23.10.2020 18:00