Chemistry, 23.07.2019 19:30 alexcarrasco5903
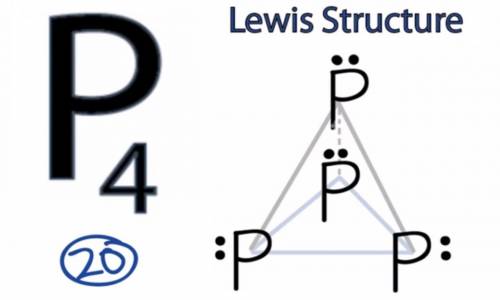
White phosphorous has the chemical formula p4(s). a p4 molecule has 20 valence electrons. draw a lewis formula for a white phosphorous molecule in which none of the atoms violates the octet rule and the formal charge on each atom is zero. there are no pi bonds in the structure.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3


Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
White phosphorous has the chemical formula p4(s). a p4 molecule has 20 valence electrons. draw a lew...
Questions


Mathematics, 23.12.2019 00:31

Physics, 23.12.2019 00:31

Chemistry, 23.12.2019 00:31

Physics, 23.12.2019 00:31



Mathematics, 23.12.2019 00:31

Mathematics, 23.12.2019 00:31


History, 23.12.2019 00:31


Mathematics, 23.12.2019 00:31


Mathematics, 23.12.2019 00:31

Mathematics, 23.12.2019 00:31

Biology, 23.12.2019 00:31


Mathematics, 23.12.2019 00:31