

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
The radioactive element carbon-14 has a half-life of 5750 years. a scientist determined that the bon...
Questions


Chemistry, 14.12.2020 23:10

Mathematics, 14.12.2020 23:10

English, 14.12.2020 23:10

History, 14.12.2020 23:10



Chemistry, 14.12.2020 23:10

Mathematics, 14.12.2020 23:10

Physics, 14.12.2020 23:10




Mathematics, 14.12.2020 23:10

English, 14.12.2020 23:10

Biology, 14.12.2020 23:10

Mathematics, 14.12.2020 23:10


History, 14.12.2020 23:10

Mathematics, 14.12.2020 23:10

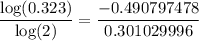
 5750 = 9374.765097 ≅ 9370 years
5750 = 9374.765097 ≅ 9370 years 

