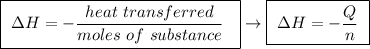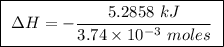Chemistry, 22.07.2019 18:00 ttwright24
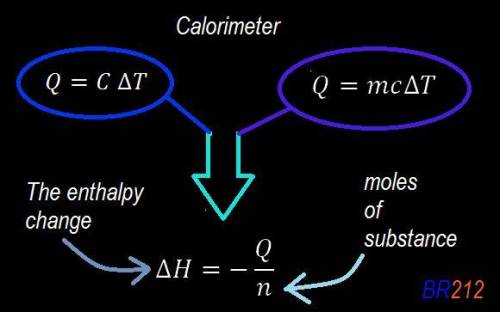
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.105-g sample of ethylene (c2h4) was burned in this calorimeter, the temperature increased by 2.14 k. calculate the energy of combustion for one mole of ethylene. a. –0.259 kj/mol b. –50.3 kj/mol c. –5.29 kj/mol d. –1.41 × 103 kj/mol e. –660 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.105-g sample of ethylene (c2h4) was bur...
Questions



Mathematics, 03.06.2021 23:10


English, 03.06.2021 23:10

Computers and Technology, 03.06.2021 23:10

Mathematics, 03.06.2021 23:10


Mathematics, 03.06.2021 23:10

Mathematics, 03.06.2021 23:10


Computers and Technology, 03.06.2021 23:10


Chemistry, 03.06.2021 23:10

Mathematics, 03.06.2021 23:10

English, 03.06.2021 23:10



Chemistry, 03.06.2021 23:20

Mathematics, 03.06.2021 23:20

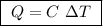
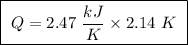
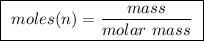
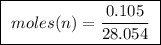
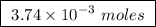 of ethylene.
of ethylene.