

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1
You know the right answer?
The density of a 50% solution of naoh is 1.525 g/ml. what volume of a solution that is 50% by weight...
Questions


Mathematics, 18.07.2019 00:30

Computers and Technology, 18.07.2019 00:30

History, 18.07.2019 00:30

History, 18.07.2019 00:30

English, 18.07.2019 00:30

Computers and Technology, 18.07.2019 00:30





History, 18.07.2019 00:30




Computers and Technology, 18.07.2019 00:30



Advanced Placement (AP), 18.07.2019 00:30

 0.4
0.4
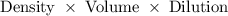
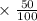
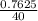
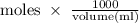
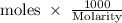
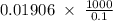 ml
ml


