
Aqueous hydrobromic acid hbr will react with solid sodium hydroxide naoh to produce aqueous sodium bromide nabr and liquid water h2o . suppose 20.2 g of hydrobromic acid is mixed with 5.7 g of sodium hydroxide. calculate the maximum mass of water that could be produced by the chemical reaction. round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3


Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1
You know the right answer?
Aqueous hydrobromic acid hbr will react with solid sodium hydroxide naoh to produce aqueous sodium b...
Questions






Mathematics, 07.04.2020 23:07

Chemistry, 07.04.2020 23:07




Computers and Technology, 07.04.2020 23:07


Mathematics, 07.04.2020 23:07







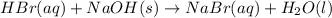
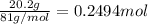
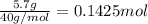
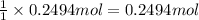 of NaOH.
of NaOH.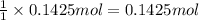 of HBr.
of HBr.

