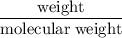Chemistry, 19.07.2019 17:20 Raekwon3232
Determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g n2o4 and 45.0 g n2h4. some possibly useful molar masses are as follows: n2o4 = 92.02 g/mol, n2h4 = 32.05 g/mol. n2o4(l) + 2 n2h4(l) → 3 n2(g) + 4 h2o(g) determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g n2o4 and 45.0 g n2h4. some possibly useful molar masses are as follows: n2o4 = 92.02 g/mol, n2h4 = 32.05 g/mol. n2o4(l) + 2 n2h4(l) → 3 n2(g) + 4 h2o(g) lr = n2o4, 105 g n2 formed lr = n2h4, 59.0 g n2 formed lr = n2o4, 45.7 g n2 formed no lr, 45.0 g n2 formed lr = n2h4, 13.3 g n2 formed

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
Determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g...
Questions

History, 27.03.2020 05:27


Mathematics, 27.03.2020 05:27

Mathematics, 27.03.2020 05:27

English, 27.03.2020 05:27





Computers and Technology, 27.03.2020 05:27


Mathematics, 27.03.2020 05:27




Mathematics, 27.03.2020 05:27




Physics, 27.03.2020 05:27

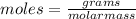 (molar mass of N2= 28)
(molar mass of N2= 28)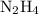 . The moles of nitrogen formed has been 2.1 mol of nitrogen.
. The moles of nitrogen formed has been 2.1 mol of nitrogen.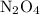 and
and