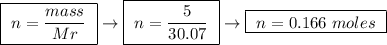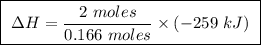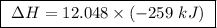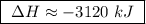Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
At constant pressure, the combustion of 5.00 g of c2h6(g) releases 259 kj of heat. what is δh for th...
Questions




Mathematics, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

Chemistry, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

History, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01


Chemistry, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01

 .
.