Chemistry, 19.07.2019 05:30 gabegabemm1
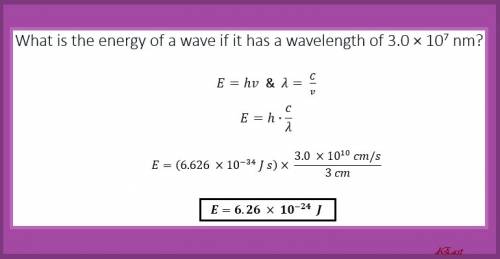
Using c=λν and e=hν (where h or planck’s constant is 6.626 *10 ⁻³⁴js). find the energy that a wavelength has if it has a wavelength of 3.000*10⁷ nm.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
You know the right answer?
Using c=λν and e=hν (where h or planck’s constant is 6.626 *10 ⁻³⁴js). find the energy that a wavel...
Questions




Social Studies, 06.07.2019 12:30


History, 06.07.2019 12:30


History, 06.07.2019 12:30









Mathematics, 06.07.2019 12:30