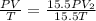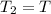Chemistry, 18.07.2019 17:40 ernestscott406
Consider a balloon that has a volume v. it contains n moles of gas, it has an internal pressure of p, and its temperature is t. if the balloon is heated to a temperature of 15.5t while it is placed under a high pressure of 15.5p, how does the volume of the balloon change?

Answers: 1


Another question on Chemistry




Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1
You know the right answer?
Consider a balloon that has a volume v. it contains n moles of gas, it has an internal pressure of p...
Questions

History, 27.09.2019 04:40

Chemistry, 27.09.2019 04:40




Mathematics, 27.09.2019 04:40



Mathematics, 27.09.2019 04:40

Mathematics, 27.09.2019 04:40




Physics, 27.09.2019 04:40

Biology, 27.09.2019 04:40



History, 27.09.2019 04:40


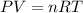
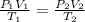
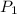 = initial pressure
= initial pressure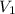 = initial volume
= initial volume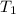 = initial temperature
= initial temperature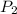 = final pressure
= final pressure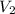 = final volume
= final volume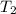 = final temperature
= final temperature