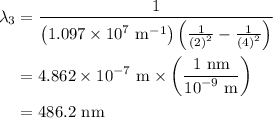Hydrogen atoms are excited by a laser to the n = 4 state and then allowed to emit. what is the maximum number of distinct emission spectral lines (lines of different wavelengths) that can be observed from this system? calculate the wavelength of the 4 to 2 transition.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
Hydrogen atoms are excited by a laser to the n = 4 state and then allowed to emit. what is the maxim...
Questions

Social Studies, 07.01.2022 01:00


English, 07.01.2022 01:00


English, 07.01.2022 01:00

Mathematics, 07.01.2022 01:00


English, 07.01.2022 01:00

Mathematics, 07.01.2022 01:00

English, 07.01.2022 01:00

Law, 07.01.2022 01:00

English, 07.01.2022 01:00

Mathematics, 07.01.2022 01:00

Mathematics, 07.01.2022 01:00



Chemistry, 07.01.2022 01:00



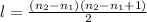
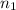 =initial energy level
=initial energy level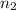 = final energy level.
= final energy level.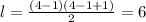
![\frac{1}{\lambda }=R[\frac{1}{n_{1}^2}-\frac{1}{n_{2}^2}]](/tpl/images/0099/1691/640ec.png)
![\frac{1}{\lambda }=1.097\times 10^{10}\times [\frac{1}{4^2}-\frac{1}{2^2}]](/tpl/images/0099/1691/e8b2e.png)
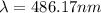 (
(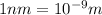 )
) .
.
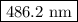 .
.
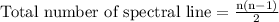 …… (1)
…… (1)

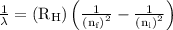 …… (1)
…… (1)
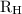 is the Rydberg constant that has the value
is the Rydberg constant that has the value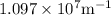 ,
, 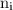 is the initial energy level of transition, and
is the initial energy level of transition, and 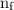 is the final energy level of transition.
is the final energy level of transition.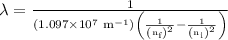 …… (2)
…… (2)