
Chemistry, 16.07.2019 19:10 girlhooper4life11
Using the table provided, which of the following answer choices represents δs for the reaction in the image? standard thermodynamic values select one: a. 18.6 j/k ·mol b. -18.6 j/k ·mol c. 550.8 j/k ·mol d. -112 j/k ·mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
You know the right answer?
Using the table provided, which of the following answer choices represents δs for the reaction in th...
Questions



English, 16.09.2019 12:10


Chemistry, 16.09.2019 12:10





Mathematics, 16.09.2019 12:10

Business, 16.09.2019 12:10


Biology, 16.09.2019 12:10

Geography, 16.09.2019 12:10



English, 16.09.2019 12:10


Computers and Technology, 16.09.2019 12:10

History, 16.09.2019 12:10

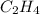 ΔS° 219.4 (J/K mol)
ΔS° 219.4 (J/K mol)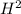 ΔS° 130.58 (J/K mol)
ΔS° 130.58 (J/K mol)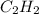 ΔS° 200.8(J/K mol)
ΔS° 200.8(J/K mol)

