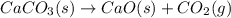Chemistry, 16.07.2019 19:00 DivineMemes420
Which statement below is not true of decomposition reactions? they usually require the addition of energy for the reaction to occur. they produce more than one product at the end of the reaction. they begin with more active reactants and end with less active products. they have one compound as the reactant at the beginning of the reaction.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
Which statement below is not true of decomposition reactions? they usually require the addition of...
Questions

Mathematics, 20.07.2019 18:10

History, 20.07.2019 18:10






Mathematics, 20.07.2019 18:10








Advanced Placement (AP), 20.07.2019 18:10

Arts, 20.07.2019 18:10

Mathematics, 20.07.2019 18:10


Mathematics, 20.07.2019 18:10