
Classify the substances according to the strongest solute-solvent interaction that will occur between the given substances and water during dissolution. match. 1. ion-dipole forces 2. dipole-dipole forces 3. hydrogen bonding 4. london dispersion forces a. alcl3 b. febr3 c. nh3 d. c2h5oh

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
Classify the substances according to the strongest solute-solvent interaction that will occur betwee...
Questions




Computers and Technology, 11.03.2021 01:30

English, 11.03.2021 01:30


Social Studies, 11.03.2021 01:30

Biology, 11.03.2021 01:30

Mathematics, 11.03.2021 01:30


English, 11.03.2021 01:30

Mathematics, 11.03.2021 01:30

History, 11.03.2021 01:30

Arts, 11.03.2021 01:30


Mathematics, 11.03.2021 01:30


Advanced Placement (AP), 11.03.2021 01:30


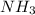 and
and 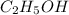 are hydrogen boding,
are hydrogen boding, 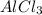 and
and 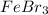 are ion-dipole forces.
are ion-dipole forces.

