

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
A0.8838-g sample of an ionic compound containing bromide ions and an unknown metal cation is dissolv...
Questions


Mathematics, 06.01.2020 05:31

Mathematics, 06.01.2020 05:31

Arts, 06.01.2020 05:31


Mathematics, 06.01.2020 05:31


Mathematics, 06.01.2020 05:31



Physics, 06.01.2020 05:31

Mathematics, 06.01.2020 05:31


Health, 06.01.2020 05:31


Mathematics, 06.01.2020 05:31



English, 06.01.2020 05:31

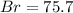 %
%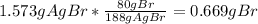
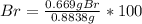 %
%

