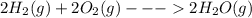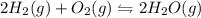Consider the chemical equation in equilibrium. 2h2(g) + o2(g) mc007-1.jpg 2h2o(g) what will happen if the pressure of the system is increased? the reaction will not change. the reverse reaction will be favored. the reaction will stop completely. the forward reaction will be favored.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 12:00
Jill is pushing a box across the floor. which represents the upward force perpendicular to the floor? a) fp b) ff c) fn d) fg
Answers: 1
You know the right answer?
Consider the chemical equation in equilibrium. 2h2(g) + o2(g) mc007-1.jpg 2h2o(g) what will happen...
Questions

Biology, 12.08.2020 05:01




Mathematics, 12.08.2020 05:01



History, 12.08.2020 05:01

English, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01

Biology, 12.08.2020 05:01


Mathematics, 12.08.2020 05:01




Social Studies, 12.08.2020 05:01



Mathematics, 12.08.2020 05:01