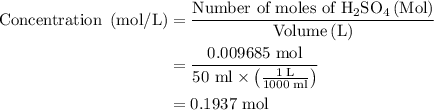Chemistry, 13.07.2019 10:40 kuddlebugsmommy
In an acid-base neutralization reaction 38.74 ml of 0.500 m potassium hydroxide (ki) reacts with 50.00 ml of sulfuric acid solution. what is the concentration of the h2so4 solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
You know the right answer?
In an acid-base neutralization reaction 38.74 ml of 0.500 m potassium hydroxide (ki) reacts with 50....
Questions

English, 07.09.2020 08:01

Physics, 07.09.2020 08:01

English, 07.09.2020 08:01

Business, 07.09.2020 08:01

Computers and Technology, 07.09.2020 08:01


Biology, 07.09.2020 08:01

Chemistry, 07.09.2020 08:01

Physics, 07.09.2020 08:01




Computers and Technology, 07.09.2020 08:01


Mathematics, 07.09.2020 08:01


Social Studies, 07.09.2020 08:01



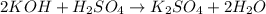
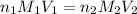
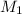 = molarity of acid
= molarity of acid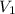 = volume of acid
= volume of acid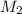 = molarity of base
= molarity of base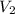 = volume of base
= volume of base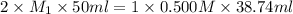
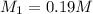
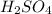 solution is 0.19M.
solution is 0.19M.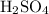 solution is
solution is  .
.
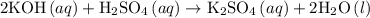
 and
and 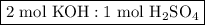
 as follows:
as follows: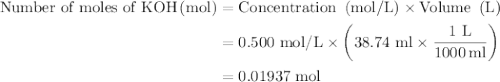
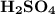 required to neutralize 0.01937 moles of
required to neutralize 0.01937 moles of