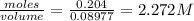Chemistry, 13.07.2019 09:30 skywil8981
A20.0 % by mass solution of phosphoric acid (h3po4) in water has a density of 1.114 g/ml at 20°c. what is the molarity of this solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
A20.0 % by mass solution of phosphoric acid (h3po4) in water has a density of 1.114 g/ml at 20°c. wh...
Questions

Mathematics, 26.01.2021 06:20

Chemistry, 26.01.2021 06:20


Social Studies, 26.01.2021 06:20

Mathematics, 26.01.2021 06:20

Health, 26.01.2021 06:20

Mathematics, 26.01.2021 06:20




English, 26.01.2021 06:20


History, 26.01.2021 06:20

Physics, 26.01.2021 06:20


Mathematics, 26.01.2021 06:20


Mathematics, 26.01.2021 06:20


Health, 26.01.2021 06:20