Chemistry, 12.07.2019 14:20 hardwick744
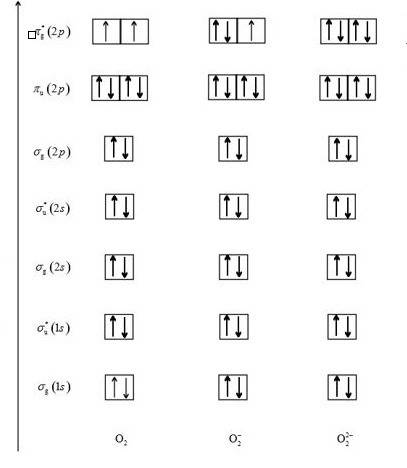
Using mo theory, predict which of the following species has the longest bond and which the strongest bond, respectively: o2, o2−, o22−

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1
You know the right answer?
Using mo theory, predict which of the following species has the longest bond and which the strongest...
Questions

Mathematics, 17.09.2020 16:01

History, 17.09.2020 16:01

Spanish, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Social Studies, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

History, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

English, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Chemistry, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01