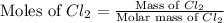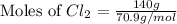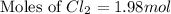Chemistry, 12.07.2019 11:00 honestty21
In the gaseous state, chlorine exists as a diatomic molecule cl2 (molar mass = 70.9 g/mol).calculate the number of moles of chlorine present in 140 g of chlorine gas.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
In the gaseous state, chlorine exists as a diatomic molecule cl2 (molar mass = 70.9 g/mol).calculate...
Questions

Mathematics, 24.10.2021 17:50

Chemistry, 24.10.2021 17:50





Mathematics, 24.10.2021 17:50

Mathematics, 24.10.2021 17:50


Mathematics, 24.10.2021 17:50

Mathematics, 24.10.2021 17:50


Mathematics, 24.10.2021 17:50




English, 24.10.2021 17:50

Mathematics, 24.10.2021 17:50


Mathematics, 24.10.2021 17:50

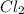 = 140 g
= 140 g