
Chemistry, 20.08.2019 03:00 dheydar9377
Agraduated cylinder contains 15.75 ml of water. a metal that weighs 31.745 g is added. the final reading is 38.55 ml. using the correct number of significant figures, determine the density of the metal = g/ml first determine volume of metal: report result to the same number of decimal places as the number with the least number of decimal places. use this volume in calculation. second determine density of metal: d=m/v report result to the same number of significant figures as the number with the least number of significant figures. do not use exponential notation in answer.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2
You know the right answer?
Agraduated cylinder contains 15.75 ml of water. a metal that weighs 31.745 g is added. the final rea...
Questions

Mathematics, 10.06.2021 01:10

Geography, 10.06.2021 01:10


Mathematics, 10.06.2021 01:10

Mathematics, 10.06.2021 01:10

Mathematics, 10.06.2021 01:10


Mathematics, 10.06.2021 01:10




World Languages, 10.06.2021 01:20


Mathematics, 10.06.2021 01:20


Mathematics, 10.06.2021 01:20


Mathematics, 10.06.2021 01:20


English, 10.06.2021 01:20

 =
= 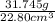 = 1.392 g/cm³
= 1.392 g/cm³

