
Chemistry, 10.07.2019 10:40 taysomoneyyy
Combustion of 25.0 g of a hydrocarbon produces 86.5 g of co2. what is the empirical formula of the compound?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1
You know the right answer?
Combustion of 25.0 g of a hydrocarbon produces 86.5 g of co2. what is the empirical formula of the c...
Questions

English, 22.05.2021 06:20



Mathematics, 22.05.2021 06:20


Geography, 22.05.2021 06:20


Mathematics, 22.05.2021 06:20

Geography, 22.05.2021 06:20

Geography, 22.05.2021 06:20

Mathematics, 22.05.2021 06:20



Mathematics, 22.05.2021 06:20




Mathematics, 22.05.2021 06:20

Mathematics, 22.05.2021 06:30

Mathematics, 22.05.2021 06:30

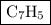 .
.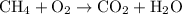
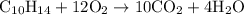
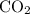 is formed as a product during combustion reactions.
is formed as a product during combustion reactions.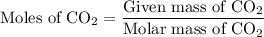 ...... (1)
...... (1)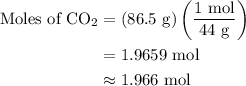
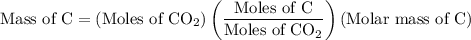 ...... (2)
...... (2)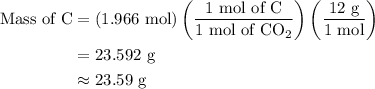
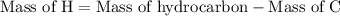 ...... (3)
...... (3)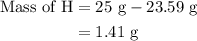
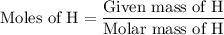 ...... (4)
...... (4)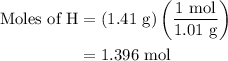
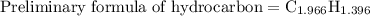

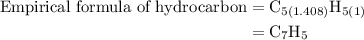
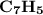 .
.


