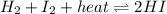Chemistry, 09.07.2019 19:00 tddreviews
Consider the chemical reaction in equilibrium. h2 + i2 + heat ⬄ 2hi what will happen to the chemical equilibrium if the temperature of the system is increased? a)the direction of the chemical equilibrium will shift to the right, favoring the forward reaction. b)the chemical equilibrium will not be affected by an increase in temperature. c)the direction of the chemical equilibrium will shift to the left, favoring the reverse reaction. d) the chemical equilibrium will be lost permanently with a change of temperature.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
Consider the chemical reaction in equilibrium. h2 + i2 + heat ⬄ 2hi what will happen to the chemic...
Questions

English, 01.08.2019 18:00


Mathematics, 01.08.2019 18:00

Mathematics, 01.08.2019 18:00


Biology, 01.08.2019 18:00




Physics, 01.08.2019 18:00






Computers and Technology, 01.08.2019 18:00




History, 01.08.2019 18:00