Chemistry, 09.07.2019 06:30 cordobamariana07
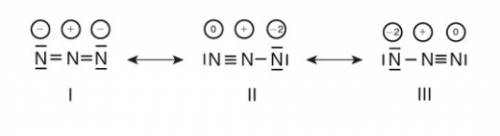
The azide ion, n−3, is a symmetrical ion, all of whose contributing structures have formal charges. draw three important contributing structures for this ion.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
The azide ion, n−3, is a symmetrical ion, all of whose contributing structures have formal charges....
Questions

Mathematics, 04.02.2021 07:00

Physics, 04.02.2021 07:00

Mathematics, 04.02.2021 07:00

English, 04.02.2021 07:00

Mathematics, 04.02.2021 07:00

Computers and Technology, 04.02.2021 07:00

Health, 04.02.2021 07:00


Chemistry, 04.02.2021 07:00



Chemistry, 04.02.2021 07:00

Physics, 04.02.2021 07:00






History, 04.02.2021 07:10