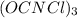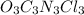Chemistry, 17.09.2019 11:30 faithb1466
The active ingredient in many types of household bleach has an empirical formula, ocncl. the molar mass is 232.41 grams per mole. what is the molecular formula of this compound? ocncl o2c2n2cl2 o3c3n3cl3 o4c4n4cl4

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1
You know the right answer?
The active ingredient in many types of household bleach has an empirical formula, ocncl. the molar m...
Questions

History, 17.04.2020 11:48








Physics, 17.04.2020 11:48

English, 17.04.2020 11:48

Geography, 17.04.2020 11:48


History, 17.04.2020 11:49




Mathematics, 17.04.2020 11:49

Chemistry, 17.04.2020 11:49

Mathematics, 17.04.2020 11:49

History, 17.04.2020 11:50