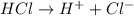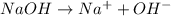1what do strong acids and strong bases have in common? a they both partially dissociate, with reverse reactions occurring. b they both dissociate completely, with little or no reverse reactions. c they both remain intact when placed in water, with no dissociation taking place. d they both dissociate completely, with reverse reactions constantly taking place.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1

Chemistry, 23.06.2019 10:30
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2
You know the right answer?
1what do strong acids and strong bases have in common? a they both partially dissociate, with reve...
Questions



Mathematics, 28.06.2019 08:30

Mathematics, 28.06.2019 08:30

Social Studies, 28.06.2019 08:30

Mathematics, 28.06.2019 08:30

Computers and Technology, 28.06.2019 08:30


History, 28.06.2019 08:30

Mathematics, 28.06.2019 08:30



History, 28.06.2019 08:30




Social Studies, 28.06.2019 08:30