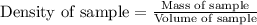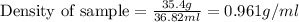Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1


Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
Remember to use the proper number of significant figures and leading zeros in all calculations. a sa...
Questions


English, 18.11.2020 03:40

Law, 18.11.2020 03:40

Mathematics, 18.11.2020 03:40

Mathematics, 18.11.2020 03:40


Mathematics, 18.11.2020 03:40



Mathematics, 18.11.2020 03:40

Arts, 18.11.2020 03:40







English, 18.11.2020 03:40

Mathematics, 18.11.2020 03:40