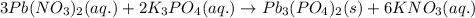Chemistry, 04.07.2019 22:50 natalie407888
When aqueous solutions of lead(ii) nitrate (pb(no3)2) and potassium phosphate (k3po4) are mixed, the products are solid lead(ii) phosphate and aqueous potassium nitrate. write the balanced chemical equation for this reaction. (include states-of-matter under the given conditions in your answer. use the lowest possible whole number

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1

Chemistry, 23.06.2019 09:00
Spaghetti sauce can be high in sodium. what is a good guideline for mg of sodium per half cup serving? a. less than 1 mg b. less than 800 mg c. less than 700 mg d. less than 400 mg
Answers: 2

Chemistry, 23.06.2019 13:30
Why hydrochloric acid neutralized first when you titrate a mixture of hcl& ch3cooh against standard sodium hydroxide
Answers: 1
You know the right answer?
When aqueous solutions of lead(ii) nitrate (pb(no3)2) and potassium phosphate (k3po4) are mixed, the...
Questions

Business, 30.10.2020 01:00

Advanced Placement (AP), 30.10.2020 01:00



Mathematics, 30.10.2020 01:00



Mathematics, 30.10.2020 01:00

Mathematics, 30.10.2020 01:00





English, 30.10.2020 01:00

Mathematics, 30.10.2020 01:00

Mathematics, 30.10.2020 01:00

Mathematics, 30.10.2020 01:00


Biology, 30.10.2020 01:00