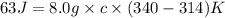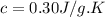Chemistry, 03.07.2019 22:00 jcultr4s3nse
Calculate the specific heat of a substance when 63j of energy are transferred as heat to an 8.0 g sample to raise it temperature from 314 k to 340 k

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
Calculate the specific heat of a substance when 63j of energy are transferred as heat to an 8.0 g sa...
Questions


Mathematics, 20.04.2021 18:20


Mathematics, 20.04.2021 18:20

Mathematics, 20.04.2021 18:20

English, 20.04.2021 18:20

Mathematics, 20.04.2021 18:20

Social Studies, 20.04.2021 18:20


Chemistry, 20.04.2021 18:20








English, 20.04.2021 18:20

Chemistry, 20.04.2021 18:20

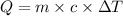
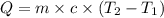
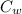 = specific heat of substance = ?
= specific heat of substance = ?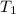 = initial temperature = 314 K
= initial temperature = 314 K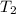 = final temperature = 340 K
= final temperature = 340 K