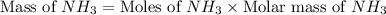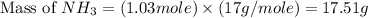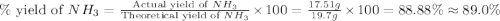Chemistry, 12.10.2019 14:30 jademckinziemea
Nitrogen gas (n2) reacts with excess hydrogen gas (h2) to produce ammonia (nh3). what is the percent yield of ammonia if the actual yield is 1.03 moles and the theoretical yield is 19.7 grams?
a. 17.5%
b. 35.0%
c. 52.3%
d. 89.0%

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1


Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1
You know the right answer?
Nitrogen gas (n2) reacts with excess hydrogen gas (h2) to produce ammonia (nh3). what is the percent...
Questions

History, 19.11.2020 01:00

Mathematics, 19.11.2020 01:00

English, 19.11.2020 01:00

English, 19.11.2020 01:00




Mathematics, 19.11.2020 01:00





Mathematics, 19.11.2020 01:00




History, 19.11.2020 01:00


Health, 19.11.2020 01:00

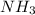 = 1.03 mole
= 1.03 mole