

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

You know the right answer?
In the reaction mg(s) + 2hcl(aq) → h2(g) + mgcl2(aq), how many moles of hydrogen gas will be produce...
Questions




Chemistry, 01.10.2019 16:50

English, 01.10.2019 16:50

Mathematics, 01.10.2019 16:50

History, 01.10.2019 16:50


Mathematics, 01.10.2019 16:50

Mathematics, 01.10.2019 16:50


Mathematics, 01.10.2019 16:50




English, 01.10.2019 16:50

Biology, 01.10.2019 16:50


History, 01.10.2019 16:50

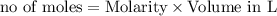
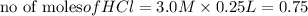
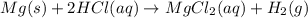
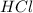 is a limiting reagent as it limits the formation of products and Mg is an excess reagent.
is a limiting reagent as it limits the formation of products and Mg is an excess reagent.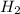
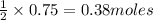 of
of 

