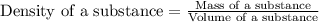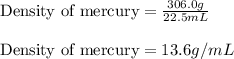Chemistry, 02.07.2019 13:10 oddoneshenchman
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml. the mercury used to fill the cylinder weighs 306.0 g. from this information, calculate the density of mercury.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 11:20
Which of the following is a pure substance? airbloodcopperwood
Answers: 2

Chemistry, 23.06.2019 13:30
The zinc within a copper-plated penny dissolves in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can reach the zinc). the reaction between the acid and the zinc 2h+(aq)+zn(s)→h2(g)+zn2+(aq) . when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 °c is 0.947 l at a total pressure of 743 mmhg . (vapor pressure of water is 23.78 mmhg at 25 °c .) what mass of hydrogen gas is collected? answer in appropriate significant figures
Answers: 3
You know the right answer?
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml. the mercury used to fi...
Questions

Mathematics, 26.08.2020 20:01

English, 26.08.2020 20:01




History, 26.08.2020 20:01

Biology, 26.08.2020 20:01



Mathematics, 26.08.2020 20:01

Physics, 26.08.2020 20:01


Mathematics, 26.08.2020 20:01


Computers and Technology, 26.08.2020 20:01

Computers and Technology, 26.08.2020 20:01

Mathematics, 26.08.2020 20:01