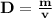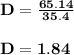Chemistry, 02.07.2019 12:00 darrell1168
Calculate the density of sulfuric acid if 35.4 ml of the acid weighs 65.14 g.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
Calculate the density of sulfuric acid if 35.4 ml of the acid weighs 65.14 g....
Questions


Chemistry, 01.12.2021 22:00

Mathematics, 01.12.2021 22:00


Mathematics, 01.12.2021 22:00




Mathematics, 01.12.2021 22:00

Mathematics, 01.12.2021 22:00




English, 01.12.2021 22:00


History, 01.12.2021 22:00

History, 01.12.2021 22:00

Mathematics, 01.12.2021 22:00

Mathematics, 01.12.2021 22:00

Mathematics, 01.12.2021 22:00

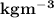 .
.