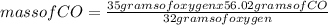When the amount of oxygen is limited, carbon and oxygen react to form carbon monoxide. how many grams of co can be formed from 35.0 grams of oxygen? 2c + o2 → 2co using 32.00 g/mole as the molecular mass of oxygen and 28.01 g/mole as the molecular mass of carbon monoxide, solve the above problem.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
When the amount of oxygen is limited, carbon and oxygen react to form carbon monoxide. how many gram...
Questions

Computers and Technology, 26.02.2020 22:05

World Languages, 26.02.2020 22:05


History, 26.02.2020 22:05


Geography, 26.02.2020 22:05

Mathematics, 26.02.2020 22:05

History, 26.02.2020 22:06

Mathematics, 26.02.2020 22:06

English, 26.02.2020 22:06


Health, 26.02.2020 22:06





Mathematics, 26.02.2020 22:06

Mathematics, 26.02.2020 22:07


Mathematics, 26.02.2020 22:07

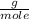 O₂: 32
O₂: 32