
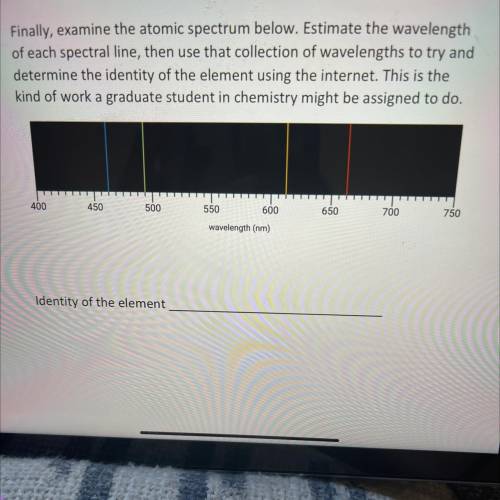
Finally, examine the atomic spectrum below. Estimate the wavelength
of each spectral line, then use that collection of wavelengths to try and
determine the identity of the element using the internet. This is the
kind of work a graduate student in chemistry might be assigned to do.
400
450
500
650
700
750
550 600
wavelength (nm)
Identity of the element

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
Finally, examine the atomic spectrum below. Estimate the wavelength
of each spectral line, then us...
Questions



History, 11.10.2020 06:01




Social Studies, 11.10.2020 06:01

Health, 11.10.2020 06:01

Mathematics, 11.10.2020 06:01

Mathematics, 11.10.2020 06:01


English, 11.10.2020 06:01

Biology, 11.10.2020 06:01


Mathematics, 11.10.2020 06:01

Mathematics, 11.10.2020 06:01




Geography, 11.10.2020 06:01



