Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2
You know the right answer?
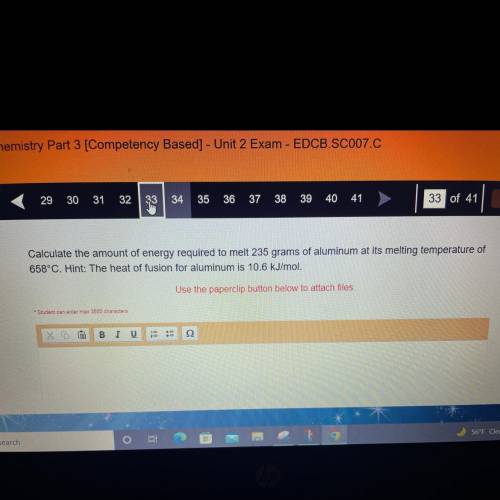
Calculate the amount of energy required to melt 235 grams of aluminum at its melting temperature of...
Questions




Mathematics, 28.06.2019 02:30




Mathematics, 28.06.2019 02:30

Mathematics, 28.06.2019 02:30

Mathematics, 28.06.2019 02:30


SAT, 28.06.2019 02:30

Computers and Technology, 28.06.2019 02:30

Physics, 28.06.2019 02:30


History, 28.06.2019 02:30

History, 28.06.2019 02:30


Mathematics, 28.06.2019 02:30

Computers and Technology, 28.06.2019 02:30