
Chemistry, 04.04.2022 19:50 shaloveywrighty5965
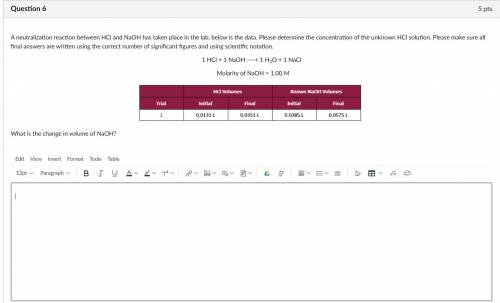
A neutralization reaction between HCl and NaOH has taken place in the lab, below is the data. Please determine the concentration of the unknown HCl solution. Please make sure all final answers are written using the correct number of significant figures and using scientific notation.
1 HCl + 1 NaOH 1 H2O + 1 NaCl
Molarity of NaOH = 1.00 M
What is the change in volume of NaOH?

Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:02
Chemistry answer as much as you can if you can't answer more then 2 don't answer
Answers: 2

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3


You know the right answer?
A neutralization reaction between HCl and NaOH has taken place in the lab, below is the data. Please...
Questions



Chemistry, 17.12.2019 18:31

Mathematics, 17.12.2019 18:31

Chemistry, 17.12.2019 18:31



Mathematics, 17.12.2019 18:31


Mathematics, 17.12.2019 18:31


Social Studies, 17.12.2019 18:31

Mathematics, 17.12.2019 18:31

English, 17.12.2019 18:31


Chemistry, 17.12.2019 18:31

History, 17.12.2019 18:31





