
Chemistry, 26.02.2022 21:40 Sadaalcala1
N2 + 3 H2 →2 NH3 How many moles of hydrogen are consumed to produce 100.g of ammonia, NH3 (17 04 g/mol)?
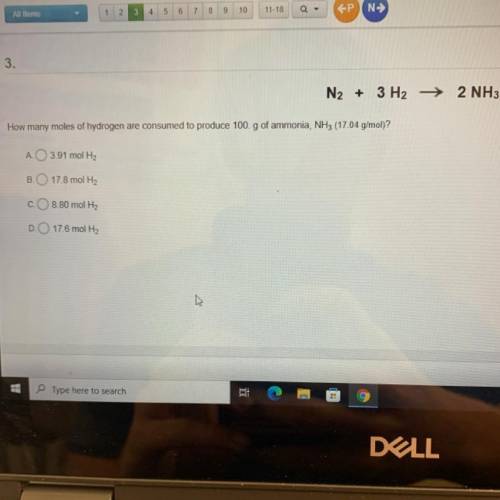

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
N2 + 3 H2 →2 NH3
How many moles of hydrogen are consumed to produce 100.g of ammonia, NH3 (17 04 g...
Questions

Mathematics, 27.10.2020 18:50

Biology, 27.10.2020 18:50

Mathematics, 27.10.2020 18:50




Mathematics, 27.10.2020 18:50


Mathematics, 27.10.2020 18:50

Biology, 27.10.2020 18:50

Mathematics, 27.10.2020 18:50


Mathematics, 27.10.2020 18:50


English, 27.10.2020 18:50

Biology, 27.10.2020 18:50

Mathematics, 27.10.2020 18:50





