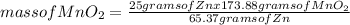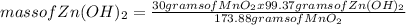Chemistry, 26.02.2022 08:50 sebastianmettsovghhk
What mass of zinc hydroxide (Zn(OH)2) will be produced if 25.0g Zn and 30.0g MnO2 react in a battery according to the following reaction: Be sure to check the limiting reactant. Zn + 2MnO2 + H2O → Zn(OH)2 + Mn2O3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
What mass of zinc hydroxide (Zn(OH)2) will be produced if 25.0g Zn and 30.0g MnO2 react in a battery...
Questions

Biology, 25.11.2020 19:20

Mathematics, 25.11.2020 19:20

Mathematics, 25.11.2020 19:20

English, 25.11.2020 19:20




Computers and Technology, 25.11.2020 19:20


Mathematics, 25.11.2020 19:20

Mathematics, 25.11.2020 19:20



History, 25.11.2020 19:20


Mathematics, 25.11.2020 19:20


Mathematics, 25.11.2020 19:20

English, 25.11.2020 19:20