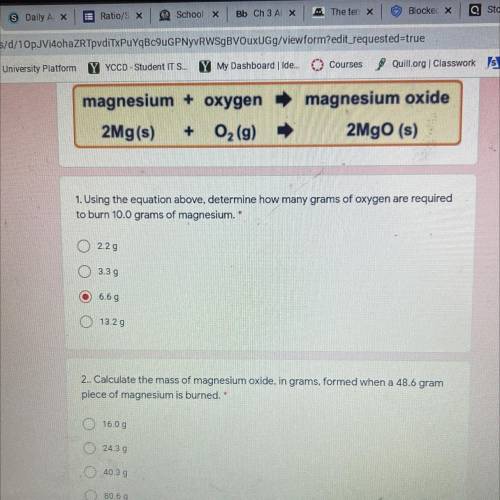
Magnesium + oxygen
2Mg(s) + O2(9)
magnesium oxide
2Mgo (s)
1. Using the equation...

Magnesium + oxygen
2Mg(s) + O2(9)
magnesium oxide
2Mgo (s)
1. Using the equation above, determine how many grams of oxygen are required
to burn 10.0 grams of magnesium. *
2.29
O 3.39
O
6.6 g
O 13.29
2.. Calculate the mass of magnesium oxide, in grams, formed when a 48.6 gram
piece of magnesium is burned. *
O 16.00
O24.39
O 40.39
80.69

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1


Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
Questions

Mathematics, 10.12.2021 17:40


Mathematics, 10.12.2021 17:40



Mathematics, 10.12.2021 17:40

Mathematics, 10.12.2021 17:40


Social Studies, 10.12.2021 17:40


Chemistry, 10.12.2021 17:40

Mathematics, 10.12.2021 17:40


English, 10.12.2021 17:40



Mathematics, 10.12.2021 17:40


Chemistry, 10.12.2021 17:40

Mathematics, 10.12.2021 17:40



