
Chemistry, 23.02.2022 14:00 BreBreDoeCCx
Write the rate law for the following reaction given that the order of A = 2, B = 1, and C = 0. A + B + C ———> D + E If the concentration of C is doubled, what will happen to the rate of the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1
You know the right answer?
Write the rate law for the following reaction given that the order of A = 2, B = 1, and C = 0. A + B...
Questions


Mathematics, 28.01.2021 20:00


Mathematics, 28.01.2021 20:00

English, 28.01.2021 20:00






Mathematics, 28.01.2021 20:00



English, 28.01.2021 20:00

Mathematics, 28.01.2021 20:00




Spanish, 28.01.2021 20:00

![\displaystyle \text{Rate} & = k[A]^2[B]](/tpl/images/2667/2602/9cfbb.png)
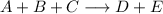
![\displaystyle \text{Rate} = k [A]^m[B]^n[C]^p](/tpl/images/2667/2602/aaf05.png)
![\displaystyle \begin{aligned} \text{Rate} & = k[A]^2[B][C]^0\\ \\ & = k[A]^2[B] \end{aligned}](/tpl/images/2667/2602/134df.png)


