
Chemistry, 21.02.2022 02:00 sofia467735
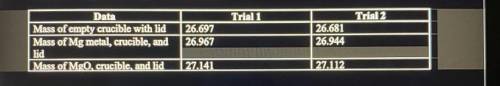
1. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for trial 1 and 2.
2. Determine the percent yield of MgO for trial 1 and 2.
3. Determine the average percent yield of MgO for both trials.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
1. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for...
Questions

Biology, 27.05.2021 22:20



Mathematics, 27.05.2021 22:20


Mathematics, 27.05.2021 22:20







Mathematics, 27.05.2021 22:20

Mathematics, 27.05.2021 22:20

Chemistry, 27.05.2021 22:20




Spanish, 27.05.2021 22:20



